The leading crosslinking technology for acrylic emulsion polymers is ambient temperature crosslinking chemistry based on diacetone acrylamide (DAAM) and adipic acid dihydrazide (ADH) monomers. This technology, known as “keto-hydrazide crosslinking,” involves the direct reaction of the pendant ketone moiety on the DAAM-acrylic polymer segment with the hydrazide moiety of the ADH, with the evaporation of water in the film-forming process.
The highest adoption of this ambient temperature, self-crosslinking technology has been in high-durability paints and coatings for architecture, wood and concrete surfaces, and more. The DAAM/ADH pair is also used in crosslinkable sizing agents, thickeners, adhesives, and sealants.
Attributes of the DAAM/ADH Crosslinking System
The DAAM/ADH crosslinking system provides several key benefits for formulators and consumers, from reducing safety concerns to improving performance properties and much more. These benefits include:
- Diacetone acrylamide uniformly copolymerizes within acrylic copolymers, creating well-dispersed pendant ketone crosslinking sites.
- Wet acrylic emulsions based on diacetone acrylamide with ADH in the aqueous phase are initially non-reactive and afford emulsions with good long-term stability during shipping and storage in retail containers (also known as "in-can" stability).
- After film coalescence, crosslinking becomes rapid at ambient temperatures, thanks to water evaporation in the drying process and a simultaneous reduction in pH arising from the loss of ammonia.
- Because crosslinking is post-coalescence, the resulting three-dimensional polymer network exhibits enhanced mechanical strength and durability as well as maximum film cohesive properties.
- Crosslinking with the keto-hydrazide chemistry enhances abrasion, scrub, stain, and blocking resistance; moisture and solvent resistance; and substrate adhesion.
With other crosslinking chemistries, premature crosslinking occurs within the emulsion particles prior to coalescence, thus retarding intermolecular diffusion between emulsion particles and resulting in weaker film products and coatings. This dynamic occurs especially when diacrylates are used in the copolymerization recipe to produce crosslinks.
In addition, DAAM and ADH are easy and safe to use. Both intermediates are formaldehyde free, unlike melamine crosslinking chemistries. DAAM and ADH are easily dissolved in warm water and in many other monomers. Finally, the by-product of the crosslinking reaction is water.
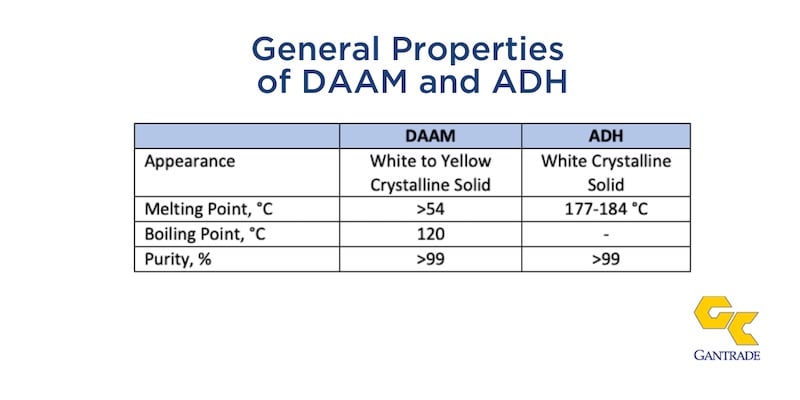
General Properties of DAAM and ADH
DAAM monomer is water-soluble and soluble in most organic solvents. The homopolymer of DAAM has a glass transition temperature (Tg) of 85 oC. and it is water soluble. ADH is moderately soluble in water.
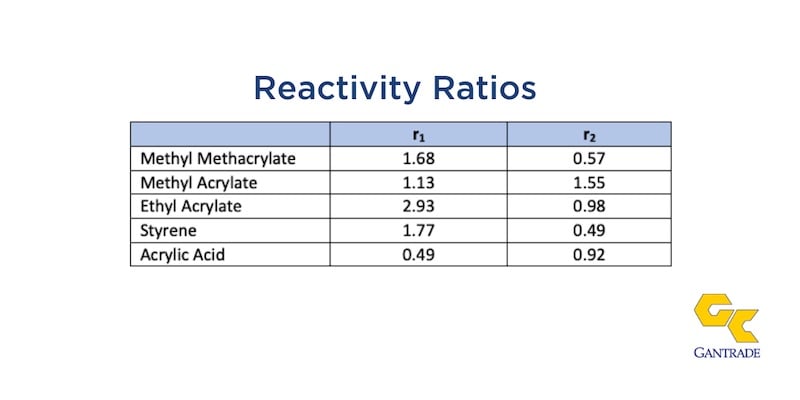
Shown in the table below are the reactivity ratios r1 and r2 for DAAM with other vinyl and acrylic monomers. The reactivity ratio for a pair of monomers is the reaction rate constant for propagation of the first monomer in a growing polymer chain to itself versus addition to the second monomer. When the reactivity ratios of comonomer pairs are similar, you’ll see random co-polymerizations. The reactivity ratios for all monomers listed show that DAAM copolymers polymerize uniformly with other monomers, affording alternating copolymers compositions closely reflecting the compositions of the monomer feeds.
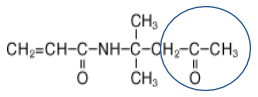
The Chemistry of DAAM/ADH Crosslinking Monomers
The circled moieties in the chemical structures below highlight where the crosslinking happens with these two molecules:
The DAAM content in the functionalized polymer is held below 5 percent to minimize chain transfer reactions, which occur with this molecule, to afford good “in-can” storage stability and to limit the influence of premature crosslinking on polymer chain diffusion in the later stages of the film-forming process.
Acrylate-DAAM copolymers post-react with ADH to form crosslinked systems under ambient conditions. ADH is a difunctional crosslinking agent which remains partitioned in the water phase outside of the emulsion particles. Here is a view of the ADH structure:
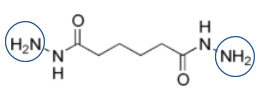
After adding ADH to a neutralized acrylic emulsion containing DAAM in the copolymer, the system remains stable when stored in the emulsion environment in the presence of ammonia or amines. The ketimine-forming reaction is acid catalyzed. Therefore, the crosslinking reaction rate is high at low pHs and almost non-existent at a pH of 8 or above. Accordingly, the functionalized polymer and ADH can co-exist with excellent in-can stability when the emulsion pH remains above neutral. The keto-hydrazide crosslinking reaction is promoted by the loss of water and concomitant volatilization of ammonia or an amine during the film-forming process. The lower volatility amines can improve in-can stability as well as regulate the rate of cross-linking during the film-forming process.
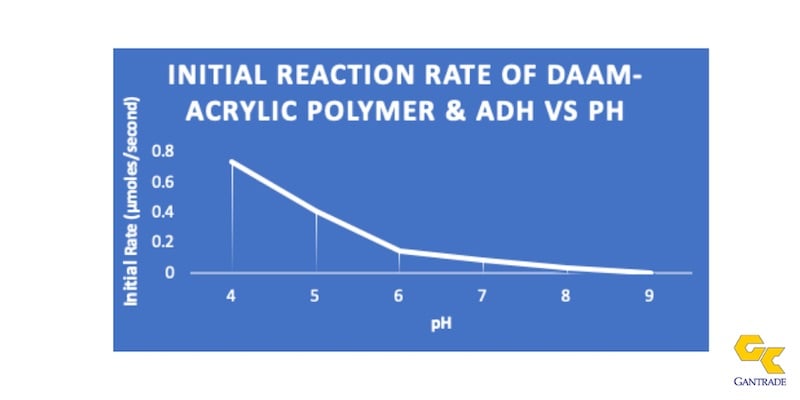
Mechanism of the Crosslinking Process
Diacetone acrylamide (DAAM) is copolymerized into an acrylic polymer to first make the emulsion polymer crosslinkable via a pendant ketone carbonyl moiety. DAAM concentration is ~1-5 wt. % of the monomer mixture. The emulsion is first neutralized with ammonia to a pH ≥ 8 and adipic dihydrazide (ADH) is then added to the emulsion as an aqueous solution. The ratio of DAAM to ADH is ~ 2.1-3.0 to 1.
The above ratio can be varied to improve in-can stability or to increase the crosslink density. Above a pH of 8, the in-can storage stability will be greater than one year, as the DAAM and ADH can coexist at the higher pH values in the emulsion state. As stated above, the ADH mainly remains partitioned in the water phase outside the emulsion particles.
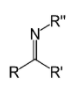
Upon drying off the water and evaporation of the ammonia, coalescence of the film occurs and the pH decreases (becomes acidic). The crosslinking reaction rate increases as the pH decreases in the later stages of film formation. The crosslinking process then takes place (acid catalyzed) with the formation of a chemical bond between the DAAM and the ADH, called a ketimine moiety, shown below. The reaction by-product is water.
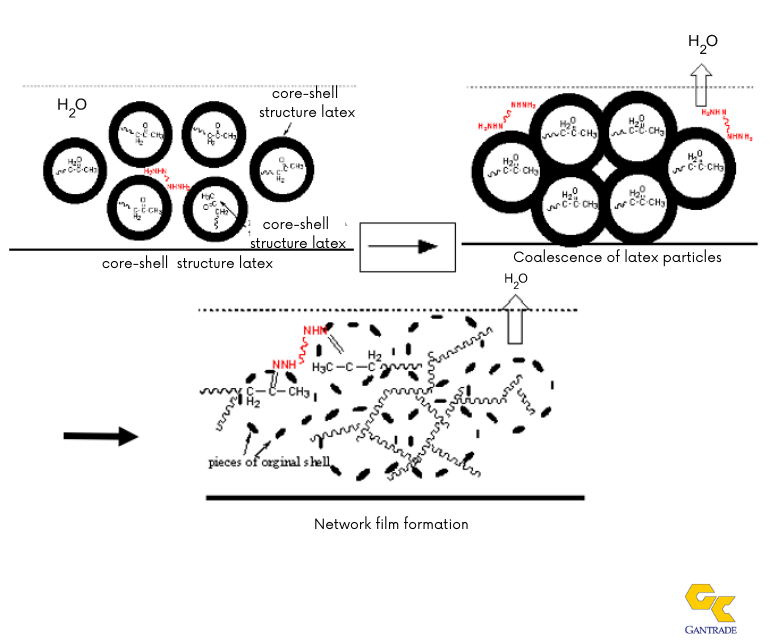
To better understand this technology, see the depiction of the coalescence and the crosslinking process below. As shown in the depiction, the ADH is mainly portioned in the aqueous phase until water is evaporated off. We also speculate that the ammonia or mono-functional amine base can tie-up the keto moiety, thus retarding the hydrazide reaction until these volatile agents are liberated in the drying process. Carboxylic acid moieties on the polymer backbone are neutralized with the presence of ammonia or an amine.
Emulsion Film Formation and Keto-Hydrazide Crosslinking Mechanism
Emulsion Copolymer Composition Options
The range of monomers that can be used with DAAM is extensive. Monomer feeds can include:
| Butyl Acrylate (BA) | |
| 2-Ethylhexyl Acrylate (2-EHA) | Glacial Acrylic Acid (GAA) |
| Methyl Methacrylate (MMA) | Glacial Methacrylic Acid (GMA) |
| Styrene | Methyl Acrylate |
The monomer composition is often dictated by the desired Tg of the polymer film, from -30 °C to > 30 °C. Reference Tg values of key monomers are provided below.
| DAAM | BA | 2EHA | MMA | St | GAA |
| 85°C | -45°C | -55°C | 105°C | 100°C | 87°C |
Emulsion Composition
To see examples of emulsion compositions you can use for copolymerization with DAAM, please consult the following recipe and tables. Key performance characteristics are shown to exemplify the attributes of DAAM-ADH crosslinked compositions.
| Reactor Composition | Weight |
| Deionized water | 90.0 g |
| Monomer Feed System A | Weight |
| Deionized water | 372.0 g |
| Rhodapex CO-436 | 8.2 g (1 weight%/polymer) |
| DAAM | 9.4 g (1.9 weight%/polymer) |
| Acrylic acid (AA) | 4.6 g (0.9% weight/polymer) |
| Styrene (St) | 74.6g (15.1 weight%/polymer) |
| Methyl methacrylate (MMA) | 80.3g (16.3 weight%/polymer) |
| Butyl acrylate (BA) | 195.2g (39.5 weight%/polymer) |
| 2-ethylhexyl acrylate (EHA) | 129.6g (26.3 weight%/polymer) |
| Initiator Feed System B | Weight |
| Deionized water | 34.6 g |
| Ammonium persulfate | 1.46 g (0.3% weight/polymer) |
| Sub-Total | (36.1 g) |
| Total Weight | 1000.0 g |
| Notes on Emulsion Properties |
|
The Applications of Diacetone Acrylamide and Adipic Acid Dihydrazide
Self-crosslinking diacetone acrylamide self-crosslinking can be used in multiple applications, the most prominent ones being durable interior and exterior paints as well as coatings for architecture, wood, and concrete surfaces. Keep on reading to discover how to formulate a DAAM-based coating as well as its performance qualities.
Architectural Coating
To create a DAAM-based coating for architectural materials, you’ll want an emulsion composition with the following compositional characteristics:
- DAAM content is 2 to 5 wt. %.
- The polymer composition can be MA/MMA/BA/DAAM.
- Tg ~ 0 ˚C.
- ADH is about 0.8 N per DAAM.
- Drying conditions: 23 ˚C; 7 days.
Below is some valuable reference information on DAAM’s coating performance (paint contained TiO2):
| DAAM Content, wt. % | 0 | 5 |
| Solvent Resistance, Steel1 Xylene Scrub | 20 | >200 |
| Pencil Hardness | 2H | 2H |
| Stain Resistance, oak plywood | Poor | Superior |
| Impact Resistance, cm | <5 | >50 |
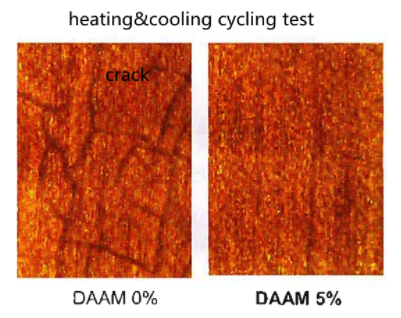
| Thermal Cooling Cycle Test2 | Poor | Superior |
1 The steel substrate was treated with zinc phosphate
2 Substrate was a fir plank. Temperature conditions: 60˚C x 2 hrs. to 20˚C x 2 hrs.
Wood Coating
For wood coatings, the DAAM emulsions can follow the guidelines below:
- DAAM content: 2 to 5 wt. %
- The polymer composition can be MA/MMA/BA/DAAM
- Tg ~ 30 °C
- ADH is 0.8 N per DAAM
- Drying conditions: 23 °C; 7 days
As you analyze diacetone acrylamide's performance in the table below, note how DAAM has a significant impact on stain resistance.
| DAAM Content, wt. % | 0 | 2 | 5 |
| Gel %, THF | 0 | 95 | 95 |
| Pencil Hardness | H | H | H |
| Impact Resistance, Steel | <50 | >50 | >50 |
| Stain Resistance: | |||
| Coloring Material | Good | Excellent | Excellent |
| Crayon | Poor | Excellent | Excellent |
| Ink (Red and Blue) | Poor | Excellent / Good | Excellent |
| Mayonnaise | Poor | Excellent | Excellent |
| Pigments | Good | Excellent | Excellent |
The stain resistance of a coating was evaluated by wiping the wood with a tissue holding the staining material and subsequently washing the wood surface with water 18 hours later.
To see how DAAM enhances the impact resistance of wood surfaces, take a look at the chart below. Notice the high-impact resistance of wood coatings with 5% DAAM versus those without DAAM-ADH crosslinking.
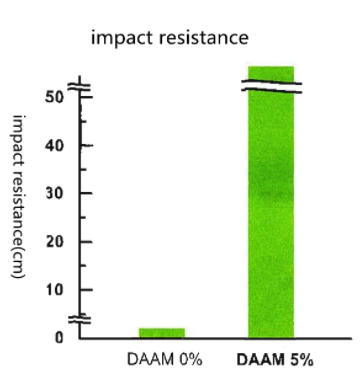
In addition, during a heating and cooling cycling test, coatings without DAAM can tend to crack. However, with a DAAM-ADH system coating, this is less likely. See the pictures blow to observe the difference.
Concrete Coating
Concrete coatings are also another application of DAAM. To prepare the coating, here are some characteristics you’ll want your emulsion to have:
- DAAM content is 2 to 5 wt. %.
- Compositions such as AA/St/MMA/BA/EHA/DAAM.
- Tg ~ -30 ˚C.
- ADH is 0.8 N per DAAM.
- Drying conditions: 23 ˚C; 7 days.
The table below provides some key information on how DAAM-ADH crosslinking impacts concrete surfaces once applied as a coating.
| DAAM Content, wt. % | 0 | 2 | 5 |
| Adhesion | Poor | Fair | Good |
| Fouling Resistance3 | |||
| Carbon Black | Poor | Fair | Good |
| Sand | Poor | Fair | Good |
| Hardness at High Temp. | Poor | Fair | Maintained |
3 Spray the Carbon Black or sand on the film surface, then scrub the surface with a brush after 30 minutes. Check residue on the film surface.
Conclusion
The keto-hydrazide crosslinking system based on the monomers DAAM and ADH represents the most advanced stage of technology for acrylic emulsion cure chemistry. DAAM levels can be up to 5% in the acrylic copolymer, but levels of about 3-4% are optimum. Formulators can optimize the polymer properties, in-can emulsion stability, film coalescence, and dried film performance by adjusting the levels and ratios of DAAM and ADH used in the final emulsion.
In addition to both safe and convenient handling, this ambient temperature crosslinking system enhances film mechanical properties, stain, water, chemical, and block resistance, toughness, impact strength, scrub resistance, adhesive properties, and more for multiple applications. This range of attribute achievement is a result of the achieving attaining high gel fractions (crosslinked polymer chains), but only after emulsion particle coalescence and the beginning of polymer-chain interdiffusion. The highest adoption of this self-crosslinking chemistry has been in high performance paints and coatings for architecture, wood, and concrete surfaces, and more.
A critical element of this crosslinking technology is “in-can” storage stability. The keto-hydrazide cure reaction is inhibited by the presence of water in the emulsion state, ammonia and amines, and a pH value above 8. The ADH mainly partitions into the aqueous phase, away from the DAAM molecule. Thus, the keto-hydrazide crosslinking does not occur until a sufficient faction of the water has evaporated and the ammonia or amine is liberated, allowing the pH to decrease below 7.
If you’re ready to leverage DAAM and ADH for your technology, Gantrade maintains good, high-quality inventories of both intermediates. In addition, we readily provide product information, technical support, and product sales information, so you can make an informed decision.
Whether you want to know more about the capabilities of DAAM-ADH systems or you want to understand the inventory options we offer, contact Gantrade to speak with one of our team members.